Copper(I) oxide

| |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Copper(I) oxide
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.883 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cu2O | |
| Molar mass | 143.09 g/mol |
| Appearance | brownish-red solid |
| Density | 6.0 g/cm3 |
| Melting point | 1,232 °C (2,250 °F; 1,505 K) |
| Boiling point | 1,800 °C (3,270 °F; 2,070 K) |
| Insoluble | |
| Solubility in acid | Soluble |
| Band gap | 2.137 eV |
| −20×10−6 cm3/mol | |
| Structure | |
| cubic | |
| Pn3m, #224 | |
a = 4.2696
| |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
93 J·mol−1·K−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−170 kJ·mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H318, H332, H410 | |
| P273, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[1] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[1] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[1] |
| Safety data sheet (SDS) | SIRI.org |
| Related compounds | |
Other anions
|
Copper(I) sulfide Copper(II) sulfide Copper(I) selenide |
Other cations
|
Copper(II) oxide Silver(I) oxide Nickel(II) oxide Zinc oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO).The compound can appear either yellow or red, depending on the size of the particles.[2] Cuprous oxide is found as the mineral cuprite. It is a component of some antifouling paints, but also has other applications including some that exploit its property as a semiconductor.
Preparation
[edit]Copper(I) oxide may be produced by several methods.[3] Most straightforwardly, it arises via the oxidation of copper metal:
- 4 Cu + O2 → 2 Cu2O
Additives such as water and acids affect the rate as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with sulfur dioxide.
Alternatively, it may be prepared via the reduction of copper(II) acetate with hydrazine:[4]
- 4 Cu(O2CCH3)2 + N2H4 + 2 H2O→ 2 Cu2O + 8 CH3CO2H + N2
Aqueous cuprous chloride solutions react with base to give the same material. In all cases, the color of the cuprous oxide is highly sensitive to the procedural details. Cu2O degrades to copper(II) oxide in moist air.
Formation of copper(I) oxide is the basis of the Fehling's test and Benedict's test for reducing sugars. These sugars reduce an alkaline solution of a copper(II) salt, giving a bright red precipitate of Cu2O.
It forms on silver-plated copper parts exposed to moisture when the silver layer is porous or damaged. This kind of corrosion is known as red plague.
Properties
[edit]Like all copper(I) compounds, cuprous oxide is diamagnetic. It does not readily hydrate to cuprous hydroxide.
Copper(I) oxide dissolves in concentrated ammonia solution to form the colourless complex [Cu(NH3)2]+, which is easily oxidized in air to the blue [Cu(NH3)4(H2O)2]2+.
Cuprous oxide is attacked by acids. Hydrochloric acid gives the chloride complex CuCl−
2. Sulfuric acid and nitric acid produce copper(II) sulfate and copper(II) nitrate, respectively.[5]
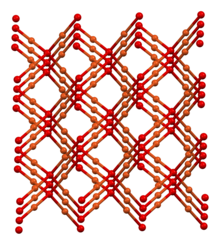
Structure
[edit]
In terms of their coordination spheres, copper centres are 2-coordinated and the oxides are tetrahedral. The structure thus resembles in some sense the main polymorphs of SiO2, but cuprous oxide's lattices interpenetrate. Cu2O crystallizes in a cubic structure with a lattice constant al = 4.2696 Å. The copper atoms arrange in a fcc sublattice, the oxygen atoms in a bcc sublattice. One sublattice is shifted by a quarter of the body diagonal. The space group is Pn3m, which includes the point group with full octahedral symmetry.
Applications
[edit]The dominant use of cuprous oxide is as a component of antifouling paints.[3]
Cuprous oxide is also commonly used as a pigment and a fungicide.
Semiconductor and related uses
[edit]Rectifier diodes based on this material have been used industrially as early as 1924, long before silicon became the standard. Copper(I) oxide is also responsible for the pink color in a positive Benedict's test. In the history of semiconductor physics, Cu2O is one of the most studied materials. Many Semiconductor applications have been demonstrated first in this material:
- Semiconductor diodes[6]
- Phonoritons ("a coherent superposition of exciton, photon, and phonon")[7][8]
The lowest excitons in Cu2O are extremely long lived; absorption lineshapes have been demonstrated with neV linewidths, which is the narrowest bulk exciton resonance ever observed.[9] The associated quadrupole polaritons have low group velocity approaching the speed of sound. Thus, light moves almost as slowly as sound in this medium, which results in high polariton densities. Another unusual feature of the ground state excitons is that all primary scattering mechanisms are known quantitatively.[10] Cu2O was the first substance where an entirely parameter-free model of absorption linewidth broadening by temperature could be established, allowing the corresponding absorption coefficient to be deduced. It can be shown using Cu2O that the Kramers–Kronig relations do not apply to polaritons.[11]
In December 2021, Toshiba disclosed a transparent cuprous oxide (Cu2O) thin-film solar cell. The cell achieved an 8.4% energy conversion efficiency, the highest efficiency ever reported for any cell of this type as of 2021. The cells could be used for high-altitude platform station applications and electric vehicles.[12]
Similar compounds
[edit]An example of natural copper(I,II) oxide is the mineral paramelaconite, Cu4O3 or CuI
2CuII
2O3.[13][14]
See also
[edit]References
[edit]- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ^ N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ^ a b Zhang, Jun; Richardson, H. Wayne (2016). "Copper Compounds". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–31. doi:10.1002/14356007.a07_567.pub2. ISBN 978-3-527-30673-2.
- ^ O. Glemser; R. Sauer (1963). "Copper (I) Oxide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2pages=1011. NY,NY: Academic Press.
- ^ D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- ^ L. O. Grondahl, Unidirectional current carrying device, Patent, 1927
- ^ Hanke, L.; Fröhlich, D.; Ivanov, A. L.; Littlewood, P. B.; Stolz, H. (1999-11-22). "LA Phonoritons in Cu2O". Physical Review Letters. 83 (21): 4365–4368. Bibcode:1999PhRvL..83.4365H. doi:10.1103/PhysRevLett.83.4365.
- ^ L. Brillouin: Wave Propagation and Group Velocity, Academic Press, New York City, 1960 ISBN 9781483276014.
- ^ Brandt, Jan; Fröhlich, Dietmar; Sandfort, Christian; Bayer, Manfred; Stolz, Heinrich; Naka, Nobuko (2007-11-19). "Ultranarrow Optical Absorption and Two-Phonon Excitation Spectroscopy of Cu2O Paraexcitons in a High Magnetic Field". Physical Review Letters. 99 (21). American Physical Society (APS): 217403. Bibcode:2007PhRvL..99u7403B. doi:10.1103/physrevlett.99.217403. ISSN 0031-9007. PMID 18233254.
- ^ J. P. Wolfe and A. Mysyrowicz: Excitonic Matter, Scientific American 250 (1984), No. 3, 98.
- ^ Hopfield, J. J. (1958). "Theory of the Contribution of Excitons to the Complex Dielectric Constant of Crystals". Physical Review. 112 (5): 1555–1567. Bibcode:1958PhRv..112.1555H. doi:10.1103/PhysRev.112.1555. ISSN 0031-899X.
- ^ Bellini, Emiliano (2021-12-22). "Toshiba claims 8.4% efficiency for transparent cuprous oxide solar cell". pv magazine. Retrieved 2021-12-22.
- ^ "Paramelaconite".
- ^ "List of Minerals". 21 March 2011.

